Many hospitals have been forced to re-open coronavirus wards. Widespread vaccination is a critical tool.
How Long Will The Covid 19 Vaccine Last Kgw Com
A fourth wave is raging in Israel fast approaching the difficult days of last January.

How long does covid 19 vaccine immunity last. What do we know about how long immunity lasts after vaccination for COVID-19. Immunity can occur naturally. The most important decision is to get a COVID-19 vaccination as soon as possible.
COVID-19 vaccines are safe and effective. Most Israelis thought the worst of COVID-19 was behind them but infection rates have doubled in the past two weeks. However these studies are ongoing and we now know that immunity actually lasts longer than we anticipated.
Remember that both vaccines were authorized in. The results provide hope that people receiving SARS-CoV-2 vaccines will develop similar lasting immune memories after vaccination. But since COVID-19 is so new experts arent sure if immunity will.
Moderna says COVID-19 vaccine immunity to stay at least a year. So how long does coronavirus vaccine immunity. We dont know how long T-cell immunity persists after COVID-19 vaccination or infection but theres evidence that T cells last for quite a while.
Studies of recovered COVID-19 patients and vaccine recipients show that immunity to the coronavirus may last anywhere from 6 months to a year or more. In April a report in The New England Journal of Medicine NEJM said that in all 33 participants who had received the Moderna vaccine during the Phase I trial protection remained high for six months after the second shot. However they will most likely have to be administered annually.
New research finds that mRNA COVID-19 vaccines provide immunity for at least 6 months. The Moderna and Pfizer-BioNTech vaccines offer immunity against COVID-19 for at least six months and might offer protection for up to two to three years. Multiple studies seem to suggest immunity declines over time though what this means is unclear Last modified on Wed 25 Aug 2021 1035 EDT With plans for the UKs Covid vaccine.
Researchers have found the immune response to the Pfizer and Moderna vaccines could last a long time. Antibodies also dont tell the whole. Studies of two of the most prominent COVID-19 vaccines suggest they remain effective for at least six months.
These study results dont mean that protection only lasts six months just that so far at the six-month checkpoint immunity is still going strong. Immunity should last for at least a year the company said in January. All currently authorized and recommended COVID-19 vaccines are safe and effective and CDC does not recommend one vaccine over another.
There is reason to think that immunity could last for several months or a couple of years at least given what we know about other viruses and what we have seen so far in terms of antibodies in patients with covid-19 and in people who have been vaccinated. It typically takes two weeks after you are fully vaccinated for the body to build protection immunity against the virus that causes COVID-19. That same month Pfizer reported that its vaccine was still highly effective at six months.
You may have side effects after vaccination but these are normal. How long does immunity from COVID-19 vaccines last. Initial data has also shown that vaccines from Moderna retain most of their effectiveness for at least six months though for how much longer has not been determined.
The immune systems of more than 95 of people who recovered from COVID-19 had durable memories of the virus up to eight months after infection. A 2004 study of individuals who were vaccinated against measles as children found strong T-cell immunity more than 30 years later. We might expect immunity to last against Covid-19 but probably not as long as measles.
Get all the facts at GoodRx. And we will probably have to vaccinate against variants. Covid-19 patients who recovered from the disease still have robust immunity from the coronavirus eight months after infection according to a new study.
The CEO of one vaccine maker said immunity may start to fade within a year. Trial data for the Pfizer-BioNTech vaccine has revealed that even after 85 days of the second vaccination dose the body still had the necessary antibodies to protect against SARS CoV-2 Covaxin has claimed its COVID-19 vaccine is capable of producing antibodies which can persist in the body for six to twelve months Oxford-AstraZenecas adenovirus vaccine being manufactured at. For those who recover from COVID-19 immunity to the virus can last at least 8 months and maybe longer research shows.
Initially we knew immunity lasted at least six months after vaccination because the first trials had six months of data at that time. One of the most pressing questions about COVID-19 vaccines is how long they can provide protection. If you are not vaccinated find a vaccine.
Here is what you need to know. The committee voted 17 to 4 to recommend authorizing the first COVID-19 vaccine in the US.
Covid Vaccine First Milestone Vaccine Offers 90 Protection Bbc News
COVID-19 Vaccination Recommendations On Dec.

Who will make the first covid vaccine. It also was featured in a fact check by The Poynter Institutes Politifact 2 which pronounced Bridles findings as false after interviewing Dr. Ugur Sahin CEO and Dr. While vaccine supplies were limited ACIP also voted on recommendations for who should be offered COVID-19 vaccine first.
The vaccine developed by Pfizer and its partner BioNTech is the first COVID-19 vaccine to be subject to a full review by the US. The Food and Drug Administration on Monday granted full approval to Pfizer and BioNTechs Covid-19 vaccine becoming the first in the US. The WHO Strategic Advisory Group of Experts SAGE on Immunization has issued Interim recommendations for the use of the inactivated COVID-19 vaccine BIBP developed by SinopharmChina National Pharmaceutical Group.
But as you can see below unlike Bridle Politifact neglected to go beyond interviewing someone with such a huge stake in the vaccine. What you need to know. If your Covid-19 vaccine requires two doses not much should change after you get your first shot experts say.
Regulator and to get an approval that puts the vaccine on par. A South African firm will begin producing the PfizerBioNTech Covid-19 vaccine the first time that the jab will be produced in Africa Pfizer has announced. It uses adenovirus-26 a.
UCHealth pharmacist Marissa Kim left prepares a dose of the Pfizer Covid-19. Özlem Türeci the husband and wife behind Covid vaccine that could change the world Credit. Multiple studies seem to suggest immunity declines over time though what this means is unclear Last modified on Wed 25 Aug 2021 0738 EDT With plans for the UKs Covid vaccine.
A COVID19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 SARSCoV2 the virus that causes coronavirus disease 2019 Prior to the COVID19 pandemic an established body of knowledge existed about the structure and function of coronaviruses causing diseases like severe acute respiratory syndrome SARS and Middle. The Biovac Institute based in Cape Town will manufacture the vaccine for distribution across Africa in a move that should help address the continents desperate need for more doses amid. Here is what you need to know.
Johnson Johnson Adenovirus vaccine JJs jab the worlds first single-shot Covid-19 vaccine was developed by its Janssen division in Belgium. The WHO Strategic Advisory Group of Experts SAGE on Immunization has issued Interim recommendations for the use of the inactivated COVID-19 vaccine Sinovac-CoronaVac developed by SinovacChina National Pharmaceutical Group. Drew Weissman 3 a UPenn scientist who is credited with helping to create the technology that enables the COVID mRNA vaccines to work.
The Sinovac COVID-19 vaccine. Focus Who invented the. 1 2020 ACIP recommended that healthcare personnel and long-term care facility residents be offered COVID-19 vaccination first Phase 1a.
The FDA issued emergency use authorization to a COVID-19 vaccine made by PfizerBioNTech the first.
All currently authorized and recommended COVID-19 vaccines. Vaccines are now widely available.
Federal Judge Upholds Indiana University S Covid 19 Vaccine Requirement
About 45 percent of Indiana residents are fully vaccinated against COVID-19 the 16th lowest rate among the states according to the Centers for.

Which covid vaccine is being used in indiana. COVID-19 vaccines went first to some of the sickest and oldest people in America. NEW BRUNSWICK NJ. Food and Drug Administration but Indiana is not adding the COVID vaccine to the list that K-12 students are required to have.
Walgreens now offers a COVID-19 vaccine for eligible recipients. These are all the. This means Pfizer has shown enough effectiveness in its vaccine and enough safety data to satisfy.
In the case of the Pfizer COVID mRNA vaccine these newly revealed documents raise additional questions about both the genotoxicity. Learn how to find a COVID-19 vaccine so you can get it as soon as you can. Reduce your risk of severe illness.
The first protein subunit COVID-19 vaccine to become available will likely come from the biotech company Novavax. Get the latest information answers to frequently asked questions about a coronavirus vaccine. Different COVID-19 Vaccines.
All COVID-19 and vaccination data used in this story are current as of August 24 2021. They dont make you immortal. Indiana sees 62000 wasted COVID-19 vaccine doses as vaccination rate slows.
Are safe are effective and. -- Booster doses of Johnson Johnsons one-shot coronavirus vaccine generated a big spike in antibodies the frontline. Do not wait for a specific brand.
Vaccine passports are digital or paper documents that show you were vaccinated against COVID-19 and could help you get into a growing number of. The COVID-19 vaccine doses developed by Pfizer-BioNTech and Moderna use a new type of vaccine an mRNA vaccine. It is the first COVID-19 vaccine to go from emergency use authorization status to full approval from the FDA.
We will provide the latest COVID-19 vaccine updates answers and information for you and your family. INDIANAPOLIS Indiana health officials have counted about 62000 doses of COVID-19 vaccines being tossed out in recent months as the number of people seeking the shots has fallen drastically. That is about 1 of the some 6 million vaccine shots that have been given.
Their risk of dying from COVID was high but. For context there have been 11489 known infections for every 100000 people nationwide. Vaccines decrease your risk of COVID-19.
Pfizers COVID-19 vaccine was fully approved by the US. Problems with blood clotting coagulation which are also common during COVID-19 disease are also reported. In most cases you do need an appointment.
ZyCoV-D approval and Vaccination for ages 12-17. IU Health is working closely with state public health officials for COVID-19 vaccine distribution. On 20 August 2021 India granted emergency use approval to the worlds first DNA based COVID-19 vaccine ZyCoV-D manufactured by Zydus Cadila for adults and children aged 12 years and above.
INDIANAPOLIS The Food and Drug Administration FDA has granted full approval to the Pfizer COVID-19 vaccine for people 16 and older. Michigan State football working towards being fully vaccinated two Big Ten programs make final five for a 2022 guard out of Iowa and Ohio State mandates COVID-19 vaccine. Brian Tabor says the approval should help Hoosiers who are still on-the-fence about getting the shot.
WASHINGTON AP The Pentagon said Monday that it will require service members to receive the COVID-19 vaccine now that the Pfizer vaccine has received full approval. A new campaign is encouraging employers in the Hoosier State to talk to their employees about receiving the COVID-19 vaccine. INDIANAPOLIS The FDAs full approval of Pfizers COVID vaccine is a big step forward says the president of the Indiana Hospital Association.
The state Department of Health following guidance from the Centers for Disease Control and Prevention CDC and the Advisory Committee on Immunization Practices ACIP recommends that people who are moderately to severely immunocompromised are especially vulnerable to COVID-19 because they are more at risk of serious prolonged illness receive an additional dose of the mRNA COVID-19. Their risk of mortality due to other causes was also high and in fact very high. This type of vaccine has been in development for about three decades but is only now being used for COVID-19.
India has only rolled out three vaccines so farCovishield COVAXIN and Sputnik V. The results of the clinical trial are.
India Biggest Buyer Of Covid 19 Vaccine With 1 6 Bn Doses Experts Say This Could Cover 60 Population
India expands its vaccine basket.
What is the covid 19 vaccine in india. Indias first domestic COVID-19 vaccine Covaxin TM developed and manufactured by Bharat Biotech International Limited in collaboration with the. The full list of ingredients for the Pfizer vaccine is. According to the statement made by the company phase III clinical trial will analyse the safety and immunogenicity of the vaccine candidate in about 360 healthy subjects in the age range of 18 to 65 years.
Since they contain no live components of the. However no such data are publicly available yet either in a. To produce its Covid-19 vaccine Novavax scientists first isolated the spike gene and then used another virus to carry the gene into moth cells where it went ahead and formed the spikes of the kind that stud the novel coronaviruss surface.
Polyethylene glycol-2000-NN-ditetradecylacetamide 12-Distearoyl-sn-glycero-3-. MRNA lipids 4-hydroxybutylazanediylbis hexane-61-diylbis 2-hexyldecanoate 2. COVAXIN Bharat Biotech The second COVID-19 Vaccine that has been approved for emergency use in India is an indigenous one which is called COVAXIN.
A COVID19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 SARSCoV2 the virus that causes coronavirus disease 2019 Prior to the COVID19 pandemic an established body of knowledge existed about the structure and function of coronaviruses causing diseases like severe acute respiratory syndrome SARS and Middle. Phosphocholine and cholesterol potassium chloride monobasic potassium. Hyderabad-based Biological E in collaboration with its strategic partners has initiated phase III clinical trial of its Covid-19 vaccine candidate in India.
The vaccine is an innovative leap for India which has been the first to approve a DNA platform shot for Covid-19 in the world. It also gave approval to Indian pharma company Cipla. These spike were then harvested and arranged as nanoparticles that are injected into the arm muscle.
05 5 Indias take on booster dose. As per the Ministry. The most important factor in preventing the spread of the Virus locally is to empower the citizens with the right information and taking precautions as.
To win the fight against the deadly coronavirus disease India began administering COVID-19 vaccines on 16 January 2021. One is Covishield the vaccine developed by Britains AstraZeneca and Oxford University which clinical trials show is about 70 effective in preventing COVID-19 and is being manufactured in India by the Serum Institute the countrys largest drugmaker. Government of India is taking all necessary steps to ensure that we are prepared well to face the challenge and threat posed by the growing pandemic of COVID-19 the Corona Virus.
Currently in India the Covishield vaccine and the Covaxin vaccine are the two available for commercial use. As the country emerges from the worlds biggest and deadliest COVID-19 outbreak scientists and policy makers say vaccinations will be key to India. This vaccine is developed by Indian Pharma major.
The shot will be brought to India through a supply agreement with homegrown vaccine maker Biological E Limited. Dr Randeep Guleria AIIMS chief said that India does not have enough data right now on the need for a third COVID-19 vaccine shot. Johnson and Johnsons single-dose COVID-19 vaccine.
India has so far given more than 260 million doses of three approved vaccines - Covishield Covaxin and Sputnik V. Covishield is the coronavirus vaccine that is made in India by the Pune-based Serum Institute of India SII. Explaining in detail about the vaccination Dr Sameer Gupta Director Cardiac Cath.
Here are the details about the vaccine which was released by. India has administered over 35 crore vaccine doses out of which 64 crore were second doses.
But can you get the vaccine with a peanut allergy. If You Are Allergic to an Ingredient in a COVID-19 Vaccine.
Verify Covid 19 Vaccine Allergic Reaction Questions Answered 11alive Com
If the answer is yes you should be referred to a board-certified allergistimmunologist for further evaluation prior to COVID-19 vaccination.

Peanut allergy covid vaccine reddit. Everyone 12 years and older is now eligible to receive a COVID-19 vaccine. Jackie Dunham CTVNewsca Writer. May 17 2021 258 AM.
According to the CDC if you have had a severe or an immediate allergic reaction of any severity within 4 hours after getting the first COVID-19 shot you should not get the second shot. People with food allergies can still receive the PfizerBioNTech COVID-19 vaccine. Despite arguments that vaccines are to blame for rising peanut allergies the claim that peanut oil is present in inoculations rests almost entirely on a.
The emergency use authorization for the Moderna vaccine is limited to adults aged 18 and older and studies are underway to test the vaccine in adolescents aged 12-17. Peanut Allergies and the COVID-19 Vaccine. Some people will get mild short-term side effects from vaccination including injection site reactions fever joint pain muscle aches fatigue headaches or worsened eczema a day after vaccination.
The American College of Allergy Asthma and Immunology ACAAI notes that individuals with allergies to medications foods inhalants insects and latex are probably no more likely than the general public to have an allergic reaction to the mRNA COVID-19 vaccines As well allergy experts Allergic Living has interviewed emphasize that there are no food proteins among the ingredients of either of the mRNA vaccines. Published Saturday December 12. Our goal is to create a safe and engaging place for users to connect over interests and passions.
Is the COVID-19 vaccine safe for a child who has a peanut and tree nut llergy. Overall the vaccine was also 85 effective in. Stock image showing a bowl of peanuts.
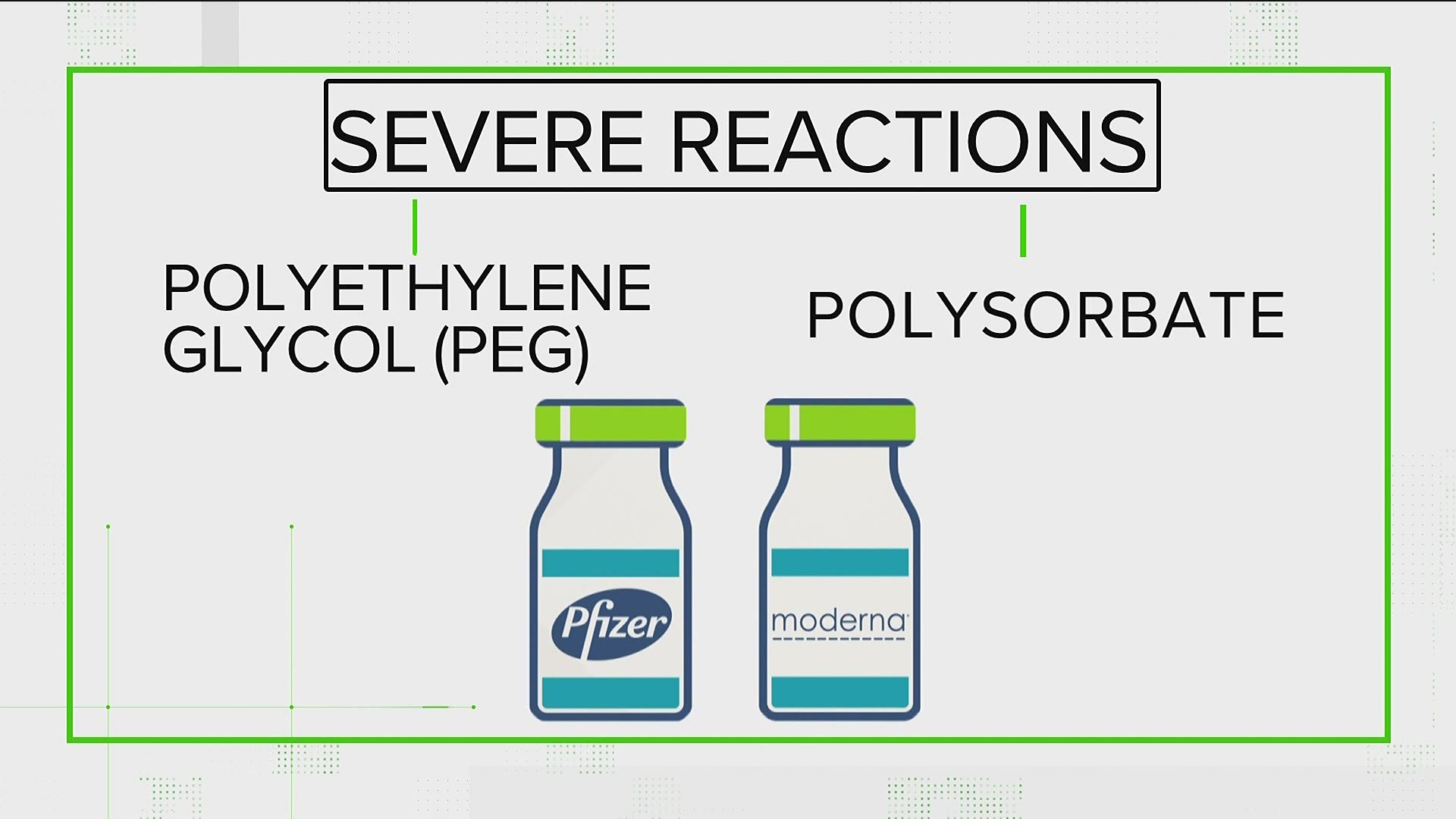
Severe allergic reactions to the Covid-19 vaccine have occurred at a rate of 2 to 5 people per million vaccinated in the United States the Centers for Disease Control and Prevention said in a. COVID-19 vaccine side effects indicate the start of an immune response not an allergic reaction. Allergists say there are ways to be vaccinated against COVID-19 safely even if you are allergic to vaccine ingredients such as PEG or polysorbate.
As the Medicines and Healthcare products Regulatory Agency MHRA has continued their close surveillance of the vaccine roll out they have now advised that individuals with a history of allergy or anaphylaxis to any food can receive any COVID-19 vaccine as long as they are not known to be allergic to any component excipient of the vaccine. Some Americans may need to avoid the COVID-19 vaccine for allergies to pharmaceuticals those with food allergies have the green light to get the shot. Health Canada warns people with allergies to Pfizer COVID-19 vaccine ingredients to not get it.
The Pfizer-BioNTech vaccine has received emergency authorization for use in individuals 12 years of age and older. Its highly recommended for people with a history of anaphylaxis that they receive their vaccines in a hospital setting and have a waiting period of 30 mins instead of 15 mins. Getting vaccinated for COVID-19 has been essential in stopping the spread of the deadly virus.
The peanut butter shot in the military is a slang term for the famous bicillin vaccination every recruit receives unless they have an allergy and can prove it. None of the currently approved UK COVID-19 vaccines. Im deathly allergic to fish shellfish peanuts and tree nuts.
The emergency use authorization for the Janssen vaccine is limited to adults aged 18 and older. Who can receive a COVID-19 vaccine. Msn back to msn.
According to professionals the chance of having a severe reaction after getting the vaccine. In clinical trials this vaccine was shown to be 66 effective in preventing moderate and severe COVID-19 disease 28 days after vaccination. Ive had both of my Pfizer covid-19 vaccines and was totally fine.
Additionally patients who experience a severe or an immediate allergic. Further to Food and Latex. In order to improve our community experience.
If you have had a severe allergic reaction or an immediate allergic reactioneven if it was not severeto any ingredient in an mRNA COVID-19 vaccine you should not get either of the currently available mRNA COVID-19 vaccines Pfizer-BioNTech and Moderna. Kagen says there is a rare chance someone will suffer an allergic reaction from the COVID-19 vaccine but there are some who should be cautious.
